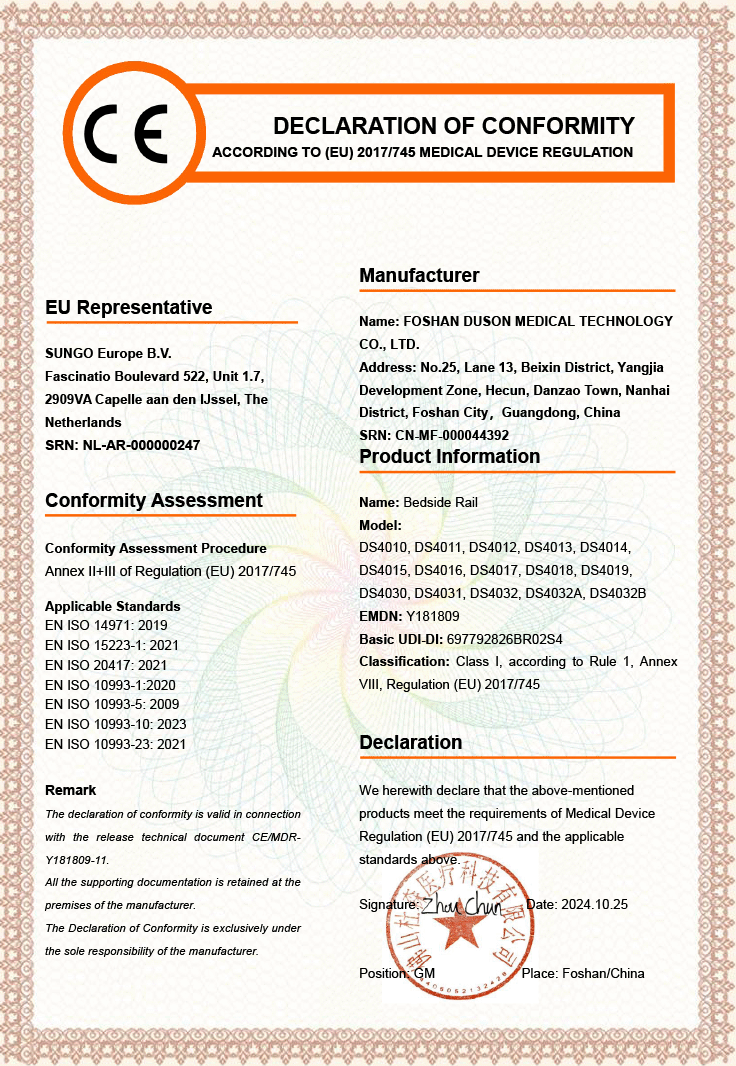
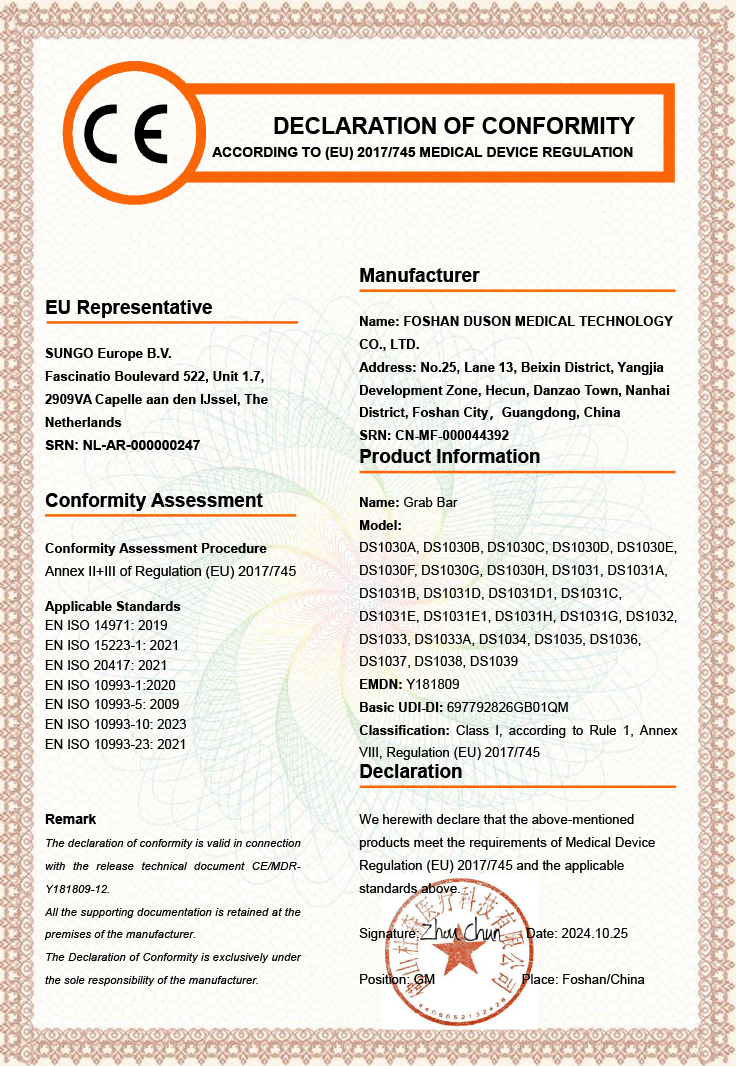
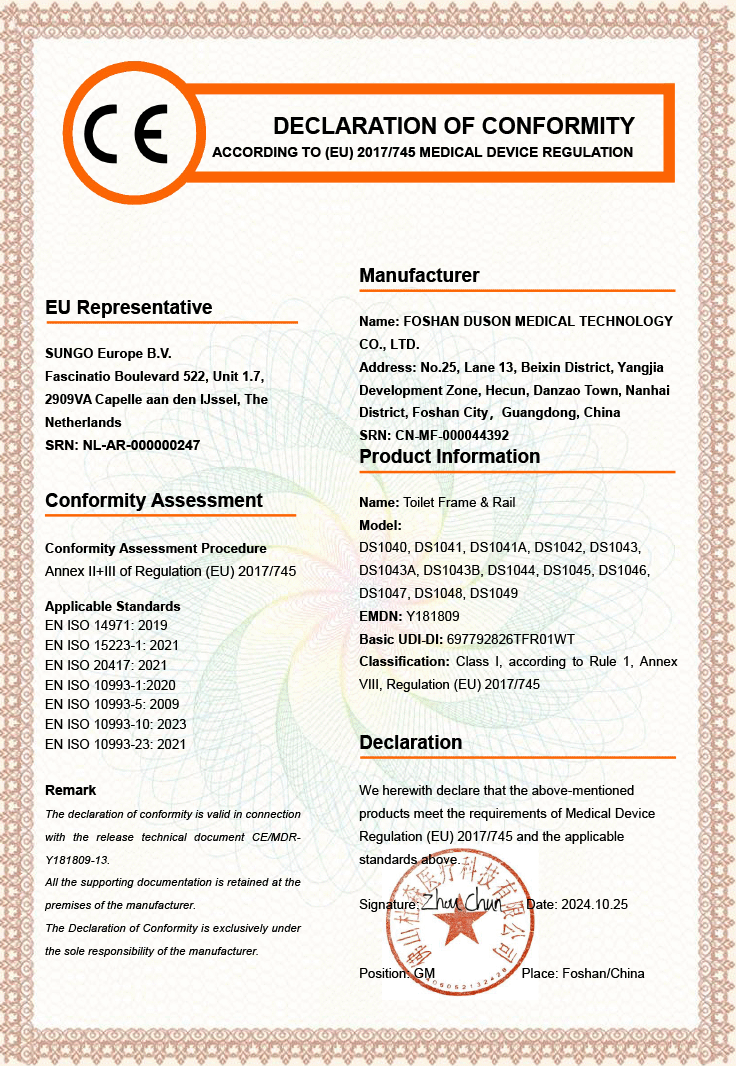
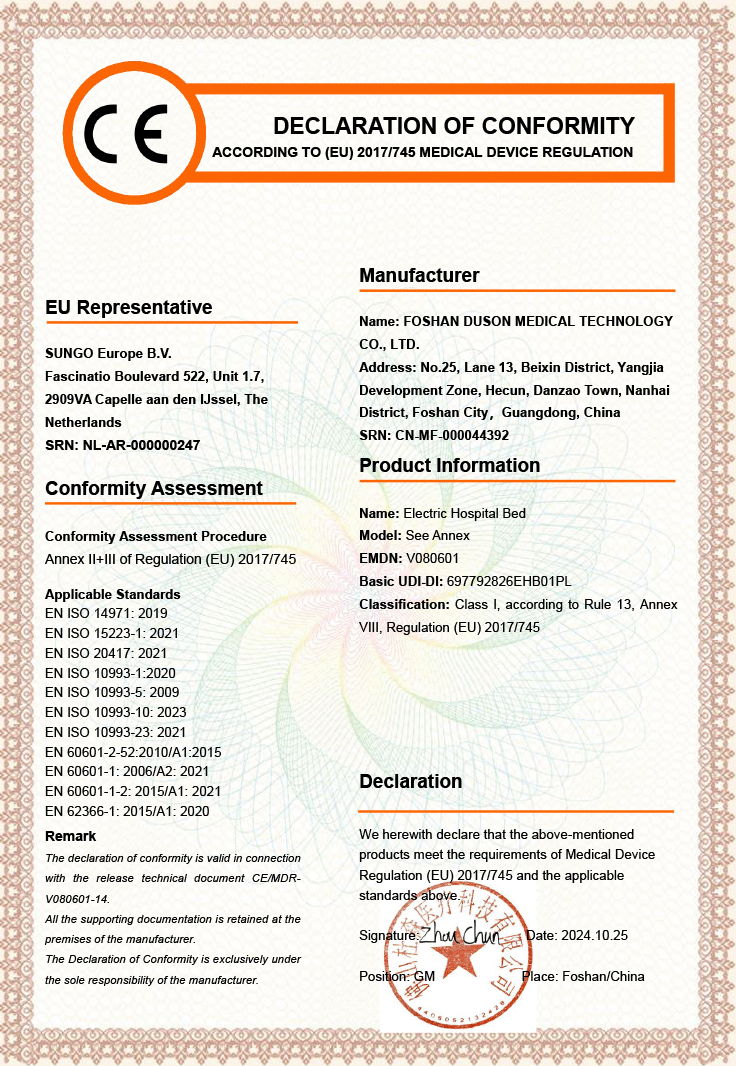
Foshan Duson Medical Technology Co., Ltd. — A Leading Manufacturer of Customized Medical Rehabilitation Products
Foshan Duson Medical Technology Co., Ltd. is located in Foshan, a major manufacturing city in Guangdong Province. Adjacent to the international hubs of Guangzhou Port and Shenzhen Port, the company enjoys exceptional transportation advantages, establishing an efficient global logistics network to ensure products are quickly delivered worldwide.
Focused on Medical Fields, Specializing in Core Segments
The company specializes in R&D and production of medical rehabilitation aids and elderly safety products, covering categories such as rehabilitation care equipment and elderly home safety devices. With mature manufacturing capabilities and a global perspective, we professionally undertake ODM (Original Design Manufacturing) and OEM (Original Equipment Manufacturing) orders. Our products have successfully entered key markets including Europe, North America, South America, Australia, Southeast Asia, and the Middle East, providing safe and reliable health product solutions to customers worldwide.
Technical Strength: From "Experience" to "Customization"
The founder of the company holds the dual roles of both "helmsman" and "technical core". With over 20 years of in-depth experience in the medical and health industry, he once served as a technical director in well-known enterprises such as Foshan Dongfang Wheelchair and Foshan Suncare Medical. During his tenure, he developed a number of mature products tailored to customer needs, thus gaining a profound understanding and precise control over the safety standards, technical details and user demands of medical rehabilitation products.